W.S. Ouriel
ABSTRACT
Hyaluronic acid (HA) is a macromolecule found in the extracellular matrix of animal tissue with a high affinity for water. Hyaluronic acid's ability to readily absorb water and form large gels has made it a subject of interest among the cosmetic industry. Hyaluronic acid's increasing popularity in the cosmetic industry is largely in part due to claims that it can hydrate the skin by absorbing 1000 times its weight in water. The claim that hyaluronic acid can hold 1000 times its weight in water is not supported in the scientific literature, nor are there any studies that examine hyaluronic acid's ability to absorb only water or examine non-crosslinked hyaluronic acid. This study examines non-crosslinked hyaluronic acid of low and high molecular weight's ability to form a saturated gel in water. It was determined that hyaluronic acid of high molecular weight was able to absorb 30 times its weight in water and hyaluronic acid of low molecular weight was able to absorb 20 times its weight in water.
INTRODUCTION
Hyaluronic acid (HA) is a macromolecule found in the extracellular matrix of animal tissue (1). Specifically, hyaluronic acid belongs to a particular class of negatively charged polysaccharides called glycosaminoglycans (GAGs) (2). A notable attribute of GAGs is their strong hydrophilic properties and their ability to form dense gels which take up large volumes in the extracellular space relative to their size (3).
Hyaluronic acid’s ability to form large gels has made it an attractive ingredient for use in dermal fillers (4). When used in dermal fillers, hyaluronic acid gels are injected into the dermis of patients to enlarge specific areas of the skin and reduce the appearance of wrinkles (5).
Hyaluronic acid has also become a popular ingredient used by the cosmetic industry for topical use. The rise in consumer demand for cosmetic products containing hyaluronic acid may be due to claims of hyaluronic acid’s water-absorbing properties, which are used in advertisements for hyaluronic acid-containing cosmetics. One such claim made by cosmetic manufacturers who sell hyaluronic acid- containing products is that hyaluronic acid can hold 1,000 times its weight in water.
There is concern with the claim that hyaluronic acid holds 1,000 times its weight in water because it is unsubstantiated in the scientific literature and appears to have no scientific basis. Despite no sound scientific support for the claims of hyaluronic acid’s ability to hold 1,000 times its weight in water, cosmetic companies continue to perpetuate the statement as fact in their advertising.
The current literature about hyaluronic acid gels is lacking in foundation to further understand its natural ability to absorb multiples of its weight in water. In particular, the published research on hyaluronic acid gels uses hyaluronic acid that has been artificially crosslinked (6). Hyaluronic acid is crosslinked to prolong its shelf life (7). Modified hyaluronic acid will form gel in a different manner than natural hyaluronic acid. The crosslinking modification will alter how hyaluronic acid interacts with the substances in its environment (8).
Another problem with the current literature is that it studies hyaluronic acid hydrogels made with substances other than water. Hyaluronic acid gels that are made with substances other than water will show significant variation in its absorbance properties (9). However, to determine hyaluronic acid’s ability to absorb water, it must be in a solution only containing water, which has not yet been studied.
This study examined non-crosslinked hyaluronic acid in a solution only containing water to determine it natural, water-absorbing capabilities. Hyaluronic acid of low and high molecular weights were examined to determine how much water hyaluronic acid binds to form a fully saturated gel. It was discovered through this study that although hyaluronic acid has a strong affinity to water that allows it to easily form gels, a fully saturated hyaluronic acid gel holds significantly less than 1,000 times its weight in water.
MATERIALS AND METHODS
HYALURONIC ACID
Hyaluronic acid powders of high molecular weight, HMW, (1.0-1.5 MDa; CAS No. 9067-32-7) and low molecular weight, LMW, (0.8-1.0 MDa; CAS No. 9067-32-7 were used for this experiment.
GEL FORMATION
Deionized water was used as the solvent to create the hyaluronic acid gels. Individual hyaluronic acid gels were made for each hyaluronic acid weight: HMW and LMW and hyaluronic acid concentration. The hyaluronic acid gels were prepared by adding 90g room temperature deionized water to a glass beaker containing hyaluronic acid of either HA HMW or HA LMW. The hyaluronic acid solution was mixed until all visible powder was dissolved, sealed, and stored at 4⁰C for 24 hours.
Initial measurements were taken with hyaluronic acid gel containing 1% hyaluronic acid. To increase the concentration of HA in the gel, the amount of hyaluronic added to the sample was increased (Table 1, Table 2).
GEL POINT AND GEL SATURATION POINT
The gel point and gel saturation points were determined by measuring the flow distance of the hyaluronic acid gels. Flow distance for each gel, hyaluronic acid HMW and hyaluronic acid LMW was measured using a GLTL Standard Consistometer (ASTM F1080/Mill Spec R-81294D). Hyaluronic acid gels were added to the consistometer’s reservoir, and the flow rate was measured after 30 seconds.
CHANGES IN HYALURONIC ACID GEL CONSISTENCY
The changes in hyaluronic acid gel’s consistency was obtained by the following calculation:
RESULTS
The consistency of hyaluronic gels changed as the concentration of hyaluronic acid increased, and this phenomenon was seen for both hyaluronic acid of low (LMW) (Fig. 1) and high molecular weights (HMW)(Fig.2) . As the concentration of hyaluronic acid increased in the water solution, the resulting gels changed in consistency, forming increasingly thickened gels. The thickened gels took longer to flow along the consistometers track, which resulted in lower (cm) consistometer readings with increased hyaluronic acid concentrations (Figs, 1, 2).
The gels containing 6% hyaluronic acid showed very little flow after 30 seconds in the consistometer, which resulted in the lowest consistometer measurements (Table 1, Table 2). The most gel flow measured in the consistometer was for hyaluronic acid gels containing 1% hyaluronic acid (Table 1, Table 2).
Hyaluronic acid HMW gels showed the lowest consistometer readings for the 1-6% concentrations of HA measured (Table 2) and hyaluronic acid LMW gels showed the highest consistometer readings for the 1-6% concentrations of HA measured (Table 1).
Table 1
Consistometer readings of hyaluronic acid (LMW) gels
_________________________________________________________
HA LMW concentration Consistometer reading
(%) (cm)
_________________________________________________________
1 20.7
2 14.8
3 12.3
4 4.20
5 2.33
6 1.37
_________________________________________________________
Table 2
Consistometer readings of hyaluronic acid (HMW) gels
_________________________________________________________
HA LMW concentration Consistometer reading
(%) (cm)
__________________________________________________________
1 17.0
2 7.43
3 4.00
4 2.30
5 1.30
6 0.63
___________________________________________________________
Fig. 1. Consistometer readings of hyaluronic acid (LMW) gel as HA concentration increases.
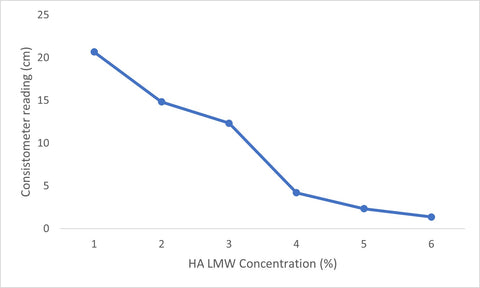
Fig. 2. Consistometer readings of hyaluronic acid (HMW) gel as HA concentration increases.

DISCUSSION
Hyaluronic acid’s water-holding capacity was dependent upon its molecular weight. This study found that Hyaluronic acid HMW gel was able to hold the most water respective to its weight at the saturation point and HA LMW was able to hold the least amount of water respective to its weight at the saturation point.
Consistency measurements were taken to determine the gel point of hyaluronic acid, and then the point at which the hyaluronic acid gel was fully saturated. The gel and saturation points would indicate how much water hyaluronic acid was able to absorb. A means of distinction between a undersaturated state, the gel point, saturation point and oversaturated point of hyaluronic acid in water had not yet been discovered the literature. Using a consistometer as the tool of measurement for gel and saturation points for this experiment showed to be the most accurate, given the non-Newtonian nature of hyaluronic acid gels (10).
Significant changes in consistency were observed when analyzing the consistometer measurements for the hyaluronic acid gels. The first significant change in consistency for hyaluronic acid LMW was observed when the concentration increased from 3% to 4%, which measured a 98.4% change in consistency. For hyaluronic acid HMW, the first significant change in consistency was measured to be 78.4% when the concentration of hyaluronic acid increased from 1-2%. The first measured abrupt change in consistency is indicative of the gel point of the solution (7).
For both hyaluronic acid LMW and hyaluronic acid HMW, a second significant change in consistency was observed, indicating the saturation point of the gel. The saturation point of the gel indicated how much water hyaluronic acid was able to absorb before becoming over-saturated. It was determined that Hyaluronic acid HMW became fully saturated at a concentration of 6% and was able to hold up to 30 times it weight in water. Hyaluronic acid LMW gel became fully saturated at a concentration of 5% and able to hold 20 times its weight in water.
The present study was limited in the amount of data points it was able to collect. Hyaluronic acid HMW and LMW gels with concentrations above 6% were overly saturated to the point that they were unable to move along the consistometer, and therefore unable to be measured. Hyaluronic acid HMW and LMW gels when in concentrations below 1% were in a semi-liquid state, and not able to be measured using the consistometer. The semi-liquid state for both types of hyaluronic acid indicated that at concentrations below 1%, a gel is unable to form. The inability of a gel to form under 1% HA, which is 100 times hyaluronic acid’s weight in water, is further evidence against the claim that hyaluronic acid can hold 1000 times its weight in water.
Data Availability Statement
Ouriel, Wendy (2022), “Hyaluronic Acid Data Set”, Mendeley Data, V1, doi: 10.17632/dt4p2c53xc.1
References
(1) Lee, D. H., Oh, J. H., & Chung, J. H. Glycosaminoglycan and proteoglycan in skin aging. J Dermatological Science, 83(3), 174-181 (2016).
(2) Juncan, A. M., Moisă, D. G., Santini, A., Morgovan, C., Rus, L. L., Vonica-Țincu, A. L., & Loghin, F. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules, 26(15), 4429 (2021).
(3) Alberts, B., Bray, D., Hopkin, K., Johnson, A., Lewis, J., Raff, M., . . . Walter, P. Essential Cell Biology, Third Edition. (Garland Science, Taylor & Francis Group LLC, New York, 2010), pp 698-699.
(4 ) Ilyin, S. O., Kulichikhin, V. G., & Malkin, A. Y. The rheological characterisation of typical injection implants based on hyaluronic acid for contour correction. Rheologica Acta, 55(3), 223-233 (2016).
(5) Tezel, A. &. The science of hyaluronic acid dermal fillers. J Cosmetic and Laser Therapy, pp. 10(1), 35-42 (2008).
(6) Xuejun, X., Netti, P. A., Ambrosio, L., Nicolais, L., & Sannino, A. Preparation and characterization of a hydrogel from low-molecular weight hyaluronic acid. J bioactive and compatible polymers, 19(1), 5-15. (2004).
(7) Andre, P. Hyaluronic acid and its use as a "rejuvenation" agent in cosmetic dermatology. Seminars in cutaneous medicine and surgery, 23(4), 218-222 (2004).
(8) Wende, F. J. Structural studies of hyaluronan hydrogels. 13-20. Uppsala: Faculty of natural resources and agricultural sciences (2019).
(9) Young, R. J., & Lovell, P. A. Introduction to polymers (CRC press, 1991), pp 306.
(10) Pisárčik, M., Bakoš, D., & Čeppan, M. Non-Newtonian properties of hyaluronic acid aqueous solution. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 97(3), 197-202 (1995).


